
ISO 80369-20 Medical Small Bore Connector Test Equipment
The Connector Assembly Device is suitable for the assembly requirements in:ISO 80369-20 annexes: B, C, D, E, F, G, H and I; as well asISO 18250-1 annexes C, D, E, F, G, H and I. This device is also suitable for carrying out the Resistance to overriding test per ISO 80369, and ISO 18250, Annex H.
- MOQ: 1
- Price: To be quoted
- Standard Packaging: safety carton pack or plywood box
- Delivery Period: 35 working days
- Payment Method: TT
- Supply Capacity: 3 sets per month
Introduction to ISO 80369-20 Test Equipment
The ISO 80369-20 Medical Small Bore Connector Test Equipment is a state-of-the-art solution designed to evaluate the performance of small-bore connectors used in medical devices. Compliant with the ISO 80369-20 standard, this equipment ensures that connectors for liquids and gases in healthcare applications meet stringent safety and performance requirements. By reducing the risk of misconnections, our test equipment plays a critical role in enhancing patient safety and ensuring compliance with international standards.
Why ISO 80369-20 Compliance Matters
The ISO 80369 series was developed to address the critical issue of medical device misconnections, particularly with small-bore connectors (inner diameter < 8.5 mm). These connectors are vital for applications such as intravenous, enteral, respiratory, and neuraxial systems. The ISO 80369-20 standard outlines common test methods to verify connector performance, including leakage, stress cracking, and resistance to axial load or torque. Our equipment is designed to perform these tests with precision, helping manufacturers meet regulatory requirements and improve device reliability.
Get Free Quote of ISO 80369-20 Medical Small Bore Connector Test Equipment
Key Benefits of Our Test Equipment
- Precision Testing: Accurately measures performance metrics like leakage (pressure decay or liquid leakage), stress resistance, and dimensional compliance per ISO 80369-20 Annexes B-I.
- Versatility: Suitable for testing connectors across various ISO 80369 standards, including ISO 80369-2 (respiratory), ISO 80369-3 (enteral), ISO 80369-5 (limb cuff), and ISO 80369-7 (intravascular/hypodermic).
- Regulatory Compliance: Supports compliance with FDA, CE, and other global regulations by adhering to ISO 80369-20 test methods.
- User-Friendly Design: Compact, durable, and requires no external power or compressed air, ensuring ease of use in laboratory settings.
- High Throughput: Semi-automatic testing capabilities increase efficiency, reducing test times while maintaining traceability and accuracy.
Features of the ISO 80369-20 Test Equipment
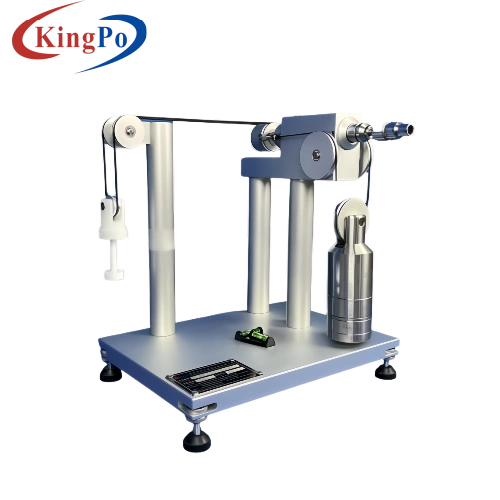
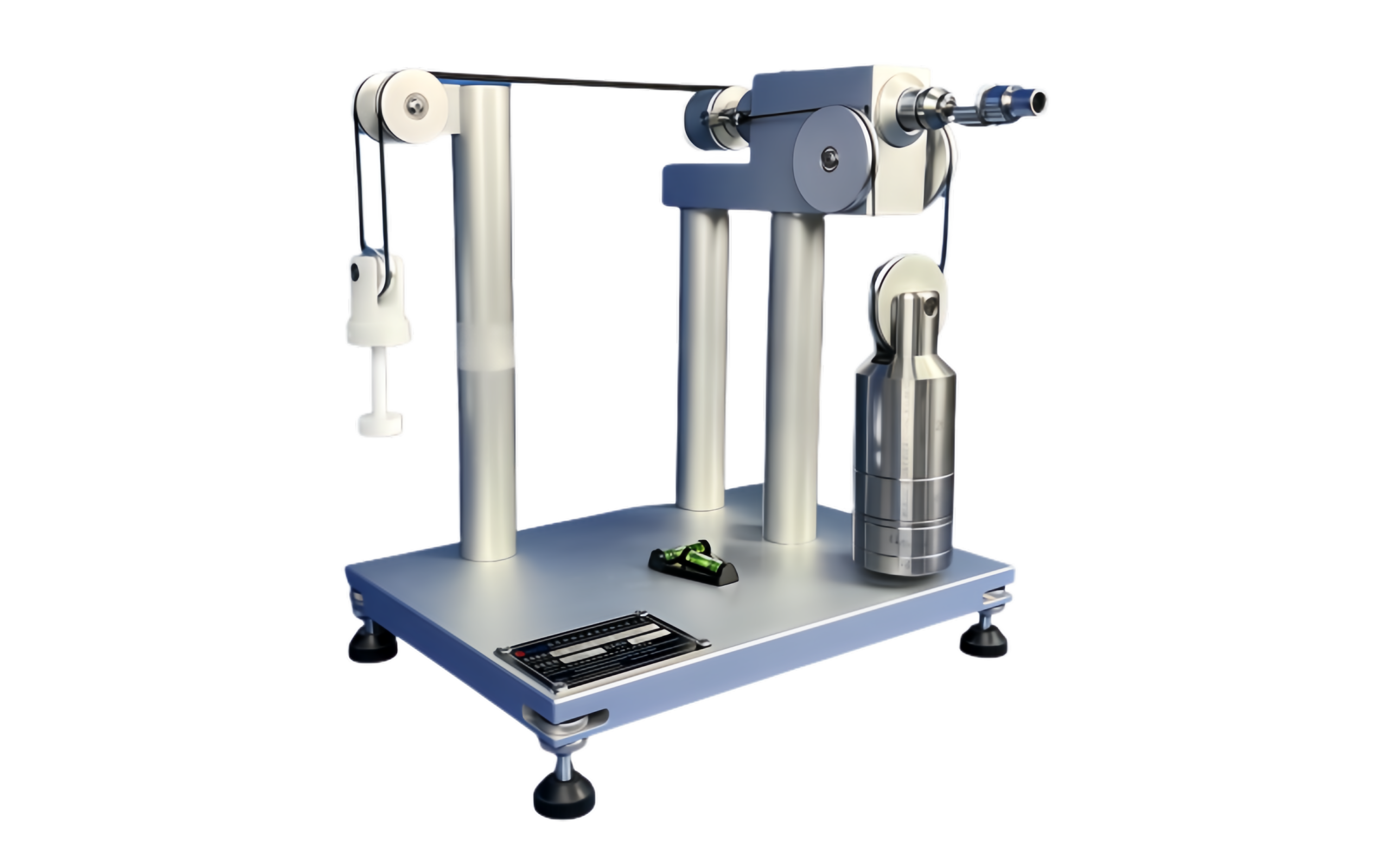
1. Connector Assembly Device
The core of our equipment is the Connector Assembly Device, crafted from anodized aluminum and hardened steel for durability. It applies precise force and torque simultaneously, as required by ISO 80369-20 Annexes B-I, to connect reference connectors with test samples. This device also supports force-only or torque-only applications, making it versatile for various test scenarios.
2. Comprehensive Test Capabilities
Our equipment supports all ISO 80369-20 test methods, including:
- Leakage Testing: Measures pressure decay (≤ 0.005 Pa*m³/sec at 300-330 kPa) or positive pressure liquid leakage per Annex B and C.
- Stress Cracking: Evaluates connector integrity under specified pressures and hold times (Annex D).
- Resistance to Separation: Tests axial load and torque resistance to ensure secure connections (Annex E and F).
- Subatmospheric Pressure Testing: Assesses air leakage during aspiration (Annex D).
3. ISO 17025 Accreditation
Our test methods are validated and accredited under ISO 17025, ensuring reliable and repeatable results. The equipment integrates with calibrated reference connectors, providing high-confidence data for regulatory submissions.
4. Compatibility with Multiple Standards
In addition to ISO 80369-20, the equipment supports testing for related standards like ISO 18250-1 (reservoir delivery systems) and ISO 80369-7 (intravascular applications), making it a versatile tool for medical device manufacturers.
Related News
China – July 15, 2025 – Kingpo Technology Development Limited, a leading manufacturer of precision testing instruments, has unveiled its latest […]