KP2021 Electrosurgical Unit Analyzer
KP2021 Electrosurgical Unit Analyzer ensures high-precision power and leakage current testing for optimal surgical safety and performance.
MOQ: 1
Price: To be quoted
Standard Packaging: 5000*600*400CM
Delivery Period: 25 working days
Payment Method: T/T
Supply Capacity: 100
Detail Information
| Place of Origin | China | Brand Name | Kingpo |
| Certification | Iso9001 Ce | Model Number | KP-2021 |
| Shipping Method: | Fedex.Door To Door Service | Working Power: | AC 220V±10% 50Hz |
| Competitive Price: | Yes | Safe Package: | Yes |
| Power: | 220V 50/60HZ | Power Supply: | 220V |
| Measure Range: | 30~50℃ | ||
| Highlight: | 50Hz Electrosurgery Analyzer, Accurate Electrosurgery Analyzer, 220V Electrosurgery Analyzer | ||
Product Description
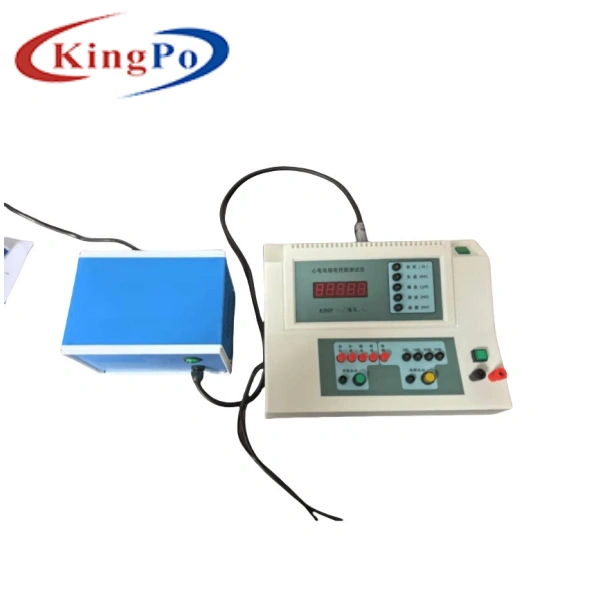
KP2021 Electrosurgical Unit Analyzer
0-500V RMS 10KHz-200MHz Medical Test Equipment
Product Overview
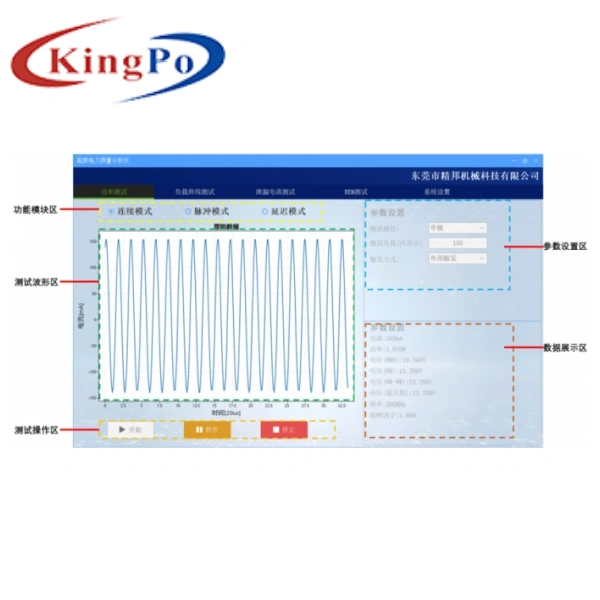
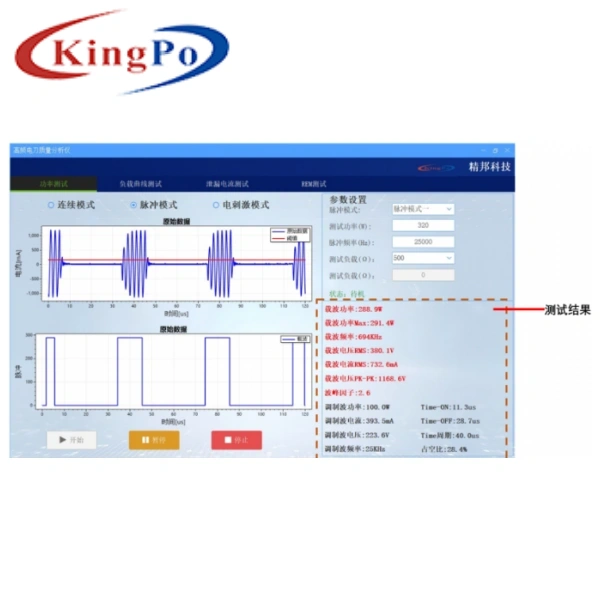
The KP2021 Electrosurgical Unit Analyzer, developed by KingPo Technology Development Limited, is designed specifically for testing the performance of electrosurgical units. It supports power testing, high-frequency leakage current testing, load curve testing, REM/CMQ testing, and is optimized for current method and voltage method testing functions to meet high-precision requirements.
Get Free Quote of KP2021 Electrosurgical Unit Analyzer
Key Features
- Measurement Range: Voltage range of 0-5kV (RMS), covering a wide range of electrosurgical devices, including those operating above 1MHz.
- High-Precision Testing: Supports testing of output current, voltage, power, crest factor, and RF leakage, compliant with IEC 60601-2-2 standards, ensuring patient safety and consistent device performance.
- Touchscreen Operation: 10-inch high-definition touchscreen for user-friendly test sequence setup and data visualization.
- Automated Testing: Streamlines testing with automated sequences, reducing test time (e.g., from 35 minutes to under 15 minutes with automation software similar to Fluke Biomedical’s Ansur).
- Data Management: Supports electronic record-keeping and data storage for long-term performance trend analysis and predictive maintenance, with USB data export to PC.
- Superior High-Frequency Testing (>1MHz): Excels in testing electrosurgical devices operating above 1MHz, such as advanced minimally invasive or dental electrosurgical equipment, with high-precision digital measurement and Signal Averaging Mode (SAM) for stable results under fluctuating high-frequency signals.
Technical Specifications
| Parameter | Technical Specifications |
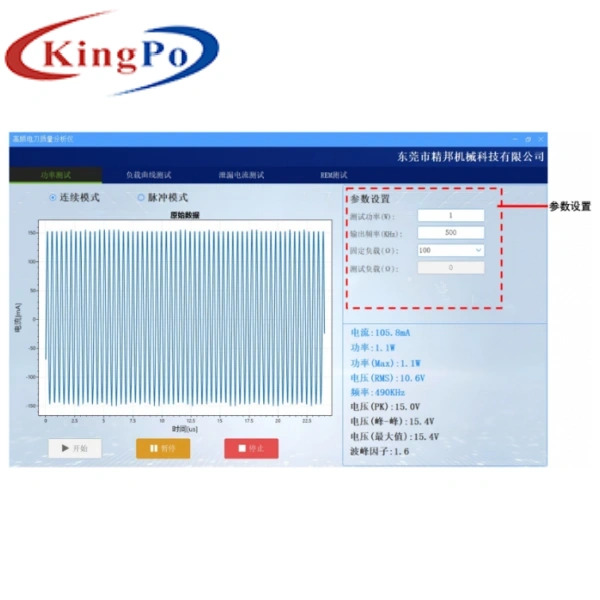
| Power | Range: 0–500W, Resolution: 0.1W, Accuracy: ≤50W: ±(5.0%*F+1)W; >50W: ±5.0% |
| Voltage (Voltage Method) | Range: 0–5kV (peak-to-peak), measured via differential voltage probe |
| Current (Current Method) | Range: 2mA–2000mA RMS, Resolution: 0.1mA, Accuracy: ±2.5% of reading |
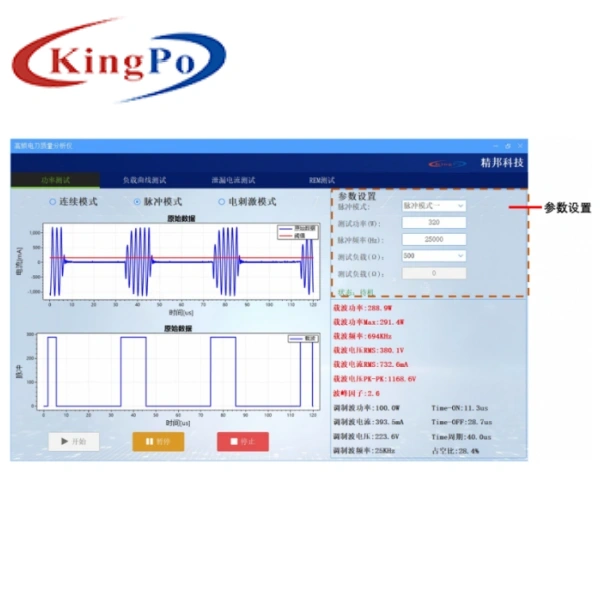
| High-Frequency Leakage Current | Range: 2mA–500mA, Resolution: 0.1mA, Accuracy: ±2.5% of reading |
| Bandwidth | 30kHz–200MHz (measurement module bandwidth) |
| Load Impedance | 0–6400 ohms, 1-ohm step, Accuracy: ±1% (≥50Ω), ±3% (≥10Ω & <50Ω) |
| Internal Load Rated Power | Max 500W (rated load 500Ω) |
| Certifications | ISO 9001 and CE certified, ensuring quality and international standard compliance. |
Testing Methods
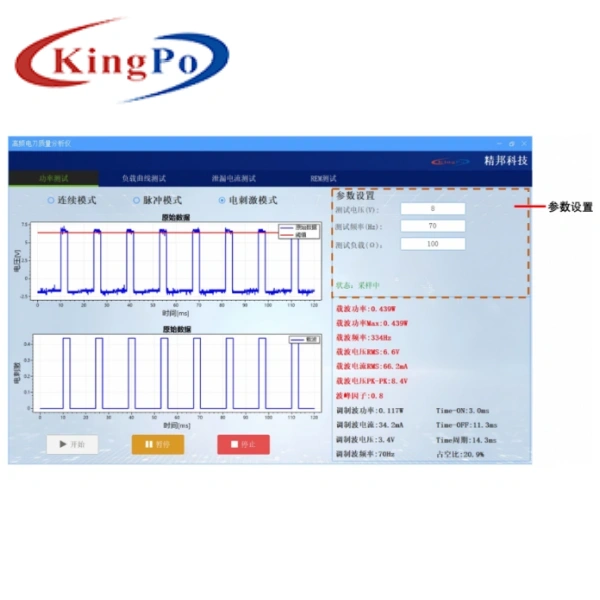
- Current Method Testing: Equipped with high-frequency current loop for precise current measurement (2mA–2000mA RMS, ±2.5% accuracy). Ideal for high-frequency leakage current testing and pulse mode analysis.
- Voltage Method Testing: Features differential voltage probe for 0–5kV peak-to-peak voltage measurement. Essential for high-voltage output analysis and safety verification.
Safety Precautions
- High-Voltage Warning: Avoid contact with connectors during operation (voltages >1000V).
- Grounding Requirements: Always connect ground port to prevent electric shock.
- Probe Usage: Ensure correct connection of current loop and voltage probe
Standards Compliance
- IEC 60601-2-2: Ensures safety and performance of high-frequency electrosurgical equipment, including RF leakage current limits and output power testing (±10% accuracy).
- ISO 17025: Calibration performed by CNAS/ilac-MRA accredited labs for traceability and accuracy.
- IEC 60601: General safety standards for medical electrical equipment, covering grounding resistance and enclosure leakage testing.
Applications
- Medical Device Manufacturers: For factory performance validation and quality control.
- Hospitals and Biomedical Engineering: For routine maintenance and safety checks to ensure reliable operation and patient safety.
- Laboratories: For R&D and performance analysis of new electrosurgical devices under varying loads.
Competitive Advantages in High-Frequency Testing (>1MHz)
Compared to competitors (e.g., Fluke Biomedical QA-ES III, Rigel Uni-Therm, Seaward), the KP2021 excels in testing electrosurgical devices operating above 1MHz:
- Wide Frequency Coverage: Supports 50Hz to 5MHz, ensuring precise testing of high-frequency ESUs with SAM for stable signal measurement, outperforming Fluke Biomedical RF303RS in high-frequency signal stability.
- Superior RF Leakage Testing: High-sensitivity module for accurate leakage current measurement at >1MHz, reducing test time and errors compared to Seaward’s manual load adjustments.
- Automated Efficiency: Built-in automation and intuitive touchscreen reduce test time to under 15 minutes, surpassing Rigel Uni-Therm’s less user-friendly interface.
- Impedance and Power Testing: Automated load adjustment and ±2.5% accuracy in power distribution tests, ideal for Argon-enhanced ESUs, unlike Fluke Biomedical’s reliance on additional accessories.
- Safety and Versatility: Supports single and bipolar modes, inert gas flow/pressure testing, and RECM/CQM for high-frequency applications, offering broader functionality than Rigel Uni-Therm.